
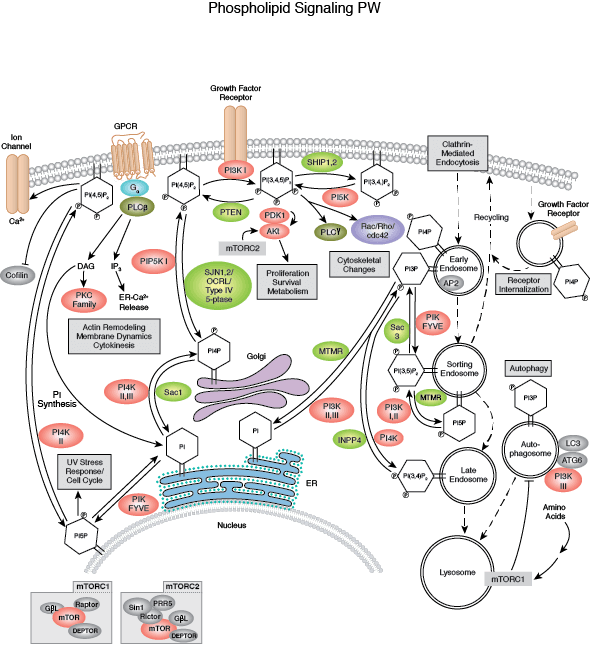
In addition, wild-type PTEN, but not mutant derivatives lacking phosphatase activity, suppresses the growth of glioblastoma cells and their tumorigenecity in nude mice ( 7– 9), confirming the functional relevance of the PTEN phosphatase domain for tumor suppression. Many cancer-related mutations have been mapped within the conserved catalytic domain of PTEN, suggesting that the phosphatase activity of PTEN is required for tumor suppressor function. PTEN has been shown in vitro to possess phosphatase activity on phosphotyrosyl, phosphothreonyl-containing substrates ( 3, 5) and more recently, on phosphatidylinositol 3,4,5-trisphosphate (PIP3), a product of phosphatidylinositol 3 (PI3) kinase ( 6). PTEN contains the sequence motif that is highly conserved in the members of the protein tyrosine phosphatase family. The tumor susceptibility gene encoding PTEN/MMAC1/TEP1 ( 1– 3) is mutated at high frequency in many primary human cancers and several familial cancer predisposition disorders ( 4). Our studies suggest that PTEN regulates the phosphatidylinositol 3,4,5,-trisphosphate and Akt signaling pathway and consequently modulates two critical cellular processes: cell cycle progression and cell survival. Akt activation increased Bad phosphorylation and promoted Pten −/− cell survival. Consequently, PTEN deficiency led to dosage-dependent increases in phosphorylation and activation of Akt/protein kinase B, a well-characterized target of the phosphatidylinositol 3 kinase signaling pathway. Inactivation of PTEN in ES cells and in embryonic fibroblasts resulted in elevated levels of phosphatidylinositol 3,4,5,-trisphosphate, a product of phosphatidylinositol 3 kinase. This accelerated G 1/S transition was accompanied by down-regulation of p27 KIP1, a major inhibitor for G 1 cyclin-dependent kinases. ES cells lacking PTEN function also displayed advanced entry into S phase. Pten −/− ES cells exhibited an increased growth rate and proliferated even in the absence of serum. To investigate the molecular basis of PTEN-mediated tumor suppression, we introduced a null mutation into the mouse Pten gene by homologous recombination in embryonic stem (ES) cells.


 0 kommentar(er)
0 kommentar(er)
